Low Carbohydrate Diet

Should I restrict my carbohydrate intake to help my body fight cancer?
Claim: Eating a low carbohydrate diet can reduce the risk of cancer and slow the growth of tumors already present by “starving” cancer cells and preventing them from multiplying.
Low Carbohydrate Diets
Low carbohydrate diets, which have become increasingly popular, vary in their level of stringency. A standard recommendation from the Academy of Nutrition and Dietetics is to consume between 50-60% of total calories from carbohydrates. For a 2,000 calories-per-day diet, 50-60% of carbohydrates is equivalent to 250-300 grams of carbohydrates per day, with the recommendation that at least 25-35 of those grams are coming from fiber. This would be equivalent to one cup of lentils plus one cup of raspberries and one cup of broccoli.
Although there is no official definition of a low-carbohydrate diet, many nutrition professionals would consider less than 40% of total calories from carbohydrates to be low. With a 2,000 calories-per-day diet, this is equivalent to less than 200 grams of carbohydrates. Very low carbohydrate consumption is at a level where ketosis occurs. For most people, this would occur at less than 50 grams of carbohydrate per day.
Paleo Diet
The Paleo Diet recommends around 20% of total calories from carbohydrates, or 100 grams per day, with adequate protein and high fat intake. The diet is based on foods that were believed to have been consumed in the Paleolithic Era, prior to agriculture and animal husbandry; therefore, it includes foods such as meat, fish, shellfish, fowl, eggs, tree nuts, vegetables, roots, fruits, berries, and mushrooms. It does not include grains, dairy, legumes, potatoes, sugar, or other refined or processed foods.
In its true form, the Paleo Diet is a lifestyle that includes exercise, restoration of sleep hygiene, and time outside. Followers of this lifestyle do so because of the belief that our pre-agricultural ancestors were largely free from modern afflictions of Western cultures, such as obesity, cancer, cardiovascular disease, and autoimmune diseases. It’s worth noting, however, that our Paleolithic ancestors had shorter lifespans, 30-35 years on average, and the risks for many of these diseases increase with age. It is suspected that many Paleolithic people died from warfare, accidents and infectious diseases, preventing the opportunity to know how their diet and lifestyle may have served them in older age.
Ketogenic Diet
The ketogenic diet is a high fat, adequate protein, and very low carbohydrate diet; only 4-5% of total calories, or 20-25 grams per day of carbohydrates are consumed. The diet forces the body to burn fats, rather than carbohydrates, for energy. This metabolic process leads to a physiological state known as ketosis. Fasting will also put the body into a state of ketosis. This diet has been studied extensively in childhood epilepsy [research].
Traditional anti-epileptic diets would begin with a medically supervised period of fasting, followed by the ketogenic diet. The diet includes high consumption of nuts, cream, butter, and other high fat foods, while excluding all grains, sugar, and starchy fruits and vegetables. The Atkins Diet is a type of ketogenic diet, and it is sometimes used as an anti-epileptic diet. The Atkins Diet is more liberal with protein, and if it is not being used for weight loss, then it allows 40-60 grams carbohydrates per day. More recently, researchers have been exploring the effects of ketosis on other health conditions, including autism, ALS, diabetes, Alzheimer’s Disease, Parkinson’s Disease, and cancer.
| Standard Diet | Paleo Diet | Ketogenic Diet | |
| Carbohydrate | 50-60% (250-300 g) |
20% (100 g) |
5% (25 g) |
| Protein | 15-20% (75-100 g) |
15-20% (75-100 g) |
15-20% (75-100 g) |
| Fat | 20-35% (45-78 g) |
60-65% (133-145 g) |
75-80% (167-178 g) |

|

|

|
Table 1. Summary of macronutrient content of various diets.
Nutrient grams based on a 2,000 calories-per-day diet.
What is Our Recommendation?
Although researchers continue to investigate the benefits of a low carbohydrate diet in cancer prevention, there is accumulating evidence that restricting carbohydrates could enhance treatment, inhibit tumor growth, extend survival, and reduce the risk of cancer development. The evidence to date does not show any adverse effects of following a low carbohydrate diet with adequate caloric intake. Although weight loss will occur if calorie intake is low, evidence suggests that with appropriate calories, a low carbohydrate diet may help to preserve muscle mass and improve quality of life even in advanced or metastatic cancers. Much of the existing evidence on safety and efficacy comes from small non-randomized studies, making it difficult to draw definitive conclusions.
In the Sugar FAQ, we discuss how high sugar consumption is associated with increased cancer risk, recurrence, and mortality. In an effort to reduce total carbohydrate intake, the first goal would be to reduce consumption of refined sugars and high glycemic carbohydrates, while balancing carbohydrate intake with good quality protein, healthy fat, and vegetables.
Studies evaluating low carbohydrate diets and cancer start to show benefits when carbohydrates are restricted to less than 20% of total daily calories. As mentioned above, this is equal to 100 grams or less, based on a 2,000 calories-per-day diet. However, the majority of the research is looking at the benefits of a ketogenic diet, meaning less than 10% of total calories, which is less than 50 grams of carbohydrates for most people. A true ketogenic diet may be much harder for people to tolerate or maintain. Anyone considering adopting this type of diet should first consult with their oncologist.
| Grams of Carbohydrates | Animal Studies | Human Studies |
| 0 | 3 | 0 |
| 10-50 (ketogenic <10%) | 14 | 7 |
| 51-100 (10-20%) | 4 | 2 |
| 101-150 (21-30%) | 0 | 0 |
| 151-200 (31-40%) | 1 | 0 |
| >200 grams (>40%) | 0 | 0 |
Table 2. Number of studies referenced in this review based on carbohydrate grams being studied.
For those who have discussed this approach with their oncologist, using online tracking tools like MyFitnessPal can be very helpful in working towards a lower carbohydrate intake. These applications will keep track of carbohydrate intake, both per meal and per day. Limiting carbohydrates to fruits, vegetables, legumes, and low glycemic grains like quinoa and wild rice will help keep your total carbohydrate intake lower. Utilizing Paleo Diet resources (cookbooks, websites, blogs) can help you sustain this level of intake.
If, however, you wish to work towards a level of ketosis, we strongly encourage you to do this with the guidance of a registered dietitian nutritionist who is experienced in ketogenic diets. This will ensure that you do it in a safe and sustainable way.
In summary, if you are interested in trying a low carbohydrate diet, here are some steps to follow:
- Avoid simple sugars and refined carbohydrates. Keep carbohydrate intake to low glycemic grains, legumes, vegetables, and fruit.
- Use an online tracking tool or app, like MyFitnessPal, to monitor your daily carbohydrate intake.
- If your carbohydrates are dropping below 100 grams per day, or if you intentionally want to work towards a ketogenic diet, talk to your oncologist and work with a registered dietitian who has experience with these types of diets.
Ketosis can be achieved by maintaining a carbohydrate intake at 50 grams or less for most people. There is some interesting research looking into other ways to achieve this. Fasting can produce ketosis, and intermittent fasting may be a way to obtain the benefits of ketosis without having to follow a strict ketogenic diet every day. Restricting calories to less than 1,000 calories per day (daily or intermittently) has also shown some potential benefit for fighting cancer; however, this approach may also lead to malnutrition for some people.
What is the Evidence?
Although much of the scientific literature on low-carbohydrate diets pertains to epilepsy, 60 relevant studies were found looking at low carbohydrate diets and cancer. Out of these studies, 34 were pre-clinical, which means that the research was done on cell lines or with animal models. Seventeen clinical studies were published, and as of January 2016, there are 13 active clinical trials.
The majority of studies published to date have investigated the effect of low-carbohydrate diets on brain tumors. A number of studies investigated its effect on prostate cancer, breast cancer, and metastatic cancer. Some studies evaluated the diet’s ability to enhance treatment. Before looking at these studies in more detail, let’s explore the physiology behind the hypothesis that restricting carbohydrate intake can influence the development and spread of cancer.
Biology:
Nobel laureate Otto Heinrich Warburg hypothesized in 1924 that cancer cells are uniquely able to generate energy in the absence of oxygen by the fermentation of sugar. This is in contrast to healthy cells, which generate energy from the oxidative breakdown of pyruvate. Healthy cells are able to produce energy not only from glucose, but also from fatty acids. Most cancer cells are not able to do this (1, 2). Refer to Figure 1 below for a visual description of this process.
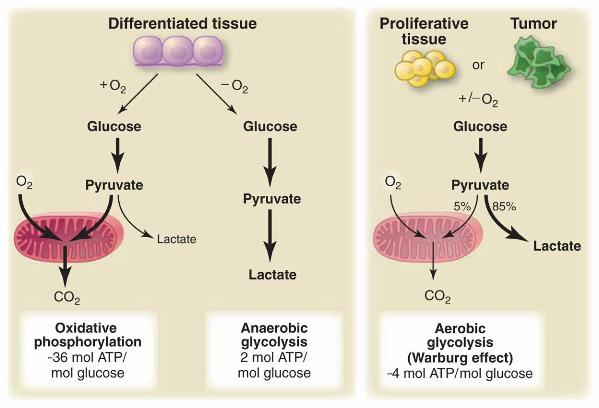
Figure 1. Schematic representation of the differences between oxidative phosphorylation, anaerobic glycolysis, and aerobic glycolysis (Warburg effect). Warburg observed that cancer cells tend to convert most glucose to lactate regardless of whether oxygen is present (aerobic glycolysis) (3).
The reliance of cancer cells on glucose could be exploited for therapeutic purposes by restricting the availability of glucose, for example through the use of a glycolytic inhibitory medication, like Metformin, or by removing most carbohydrate from the diet. By limiting the availability of glucose as a fuel source, insulin-like growth factor is also impacted. As mentioned in the Sugar FAQ, there are studies exploring the use of a glycolytic inhibitory medication along with the role of insulin and cancer.
There are varying degrees of carbohydrate restriction. Most of the studies have looked at the role of very low carbohydrate (ketogenic) diets and the ability to induce metabolic changes that no longer allow the survival of the cancer cell.
An in vitro study, looking at whether a ketogenic diet might selectively impair energy metabolism in tumor cells, found that glioma cells were incapable of compensating for glucose restriction by metabolizing ketone bodies (4). This suggests a potential disadvantage of tumor cells compared to normal cells, which can use ketone bodies for energy production.
In addition to the interference with energy production, researches are learning that ketogenic diets can also interfere with gene expression and the expression of ketolytic enzymes. One study in particular looked at the expression of two key enzymes involved in ketone metabolism in 27 patients with either glioblastoma or anaplastic glioma (5). Either absent or markedly decreased expression of these two enzymes was observed in many of the tumors, but not all. For those tumors that did express a lack or decrease of enzyme expression, it is hypothesized that a ketogenic diet could have a positive therapeutic benefit of starving those cells.
The majority of human tumors express mutant forms of the gene called p53 (6). This promotes a gain of oncogenic functions and has been correlated with disease progression, resistance to therapy, and an overall unfavorable prognosis. In vivo studies have shown that glucose restriction, induced by diet, blunts mutant p53 gene expression and oncogenic activity relative to a normal diet. Depletion of p53 leads, in turn, to an increase in autophagic activity and cell death.
Another study looking at gene expression and the ketogenic diet found that the ketogenic diet induced an overall reversal of mutant gene expression to patterns seen in non-tumor specimens (7). They also found that many genes involved in modulating reactive oxygen species (ROS) levels, as well as oxidative stress, were altered in tumor cells along with reduced expression of genes involved in signal transduction from growth factors known to be involved in glioma growth.
As mentioned above, the majority of the research being conducted in this area is evaluating the effects on brain, prostate and breast cancers. Woven into the different types of cancer is information on treatment enhancement. We will look at the work in each of these categories separately, then we will summarize what we found pertaining to other cancers, including metastases and advanced cancers.
Brain Tumor:
Mouse models of glioblastoma demonstrate treatment enhancement when mice were fed a ketogenic diet in combination with chemotherapy and/or radiation (8, 9). Additionally, studies showed that mice fed a ketogenic diet, some with a liquid ketogenic supplement and calorie restriction, have prolonged survival and significantly decreased tumor growth (9, 10, 11, 12). These studies provide a promising backdrop for the human trials.
A study published in 2014 reported results of a retrospective review of 53 patients being treated for glioblastoma with concurrent chemo-radiotherapy and adjuvant chemotherapy (13). Of these 53 patients, six followed a ketogenic diet during treatment. The diet was well tolerated and without any adverse effects. Serum glucose levels were significantly lower for those on the ketogenic diet than for those on a standard diet, despite the use of high dose steroids.
In 2014, researchers published data on the ERGO Trial (14), which was a pilot, non-randomized feasibility study (n=20) of a ketogenic diet in recurrent glioblastoma. The results suggest that although the ketogenic diet alone does not seem to extend median survival, when combined with chemotherapy, there was enhanced progression-free survival. These findings were seen again in another pilot study (n=2) published in 2015 (15). Although no benefit was seen from following a ketogenic diet alone, remission rates were improved when the diet was used in combination with treatment. Both of these studies demonstrated the safety of following a ketogenic diet. There are currently several active clinical trials looking at the potential benefit of using a ketogenic diet or modified Atkins Diet in patients with brain tumors.
Prostate Cancer:
In mouse models of prostate cancer, researchers have been evaluating varying amounts of carbohydrate intake. In 2008, researchers were able to show that mice on a no-carbohydrate diet (0 grams) had significantly reduced tumor growth and prolonged survival versus mice on a 72% carb diet (standard) or 44% carb diet (low) (16). Similar results were found in other studies (17). Another study showed that mice following a no-carbohydrate diet had significantly prolonged survival, as well as lower levels of inflammation, insulin, and obesity, along with increased apoptosis of cancer cells (18).
Although these findings are interesting, they do not readily transfer to humans, given the difficulties of following a 0 gram carbohydrate diet. In 2010, another study showed that mice fed a 10-20% carbohydrate diet had similar survival to mice consuming a 0% carbohydrate diet (19). Two more studies showed similar results, and actually one determined that mice on a 20% carbohydrate diet had the slowest rate of tumor growth (20, 21).
So far, we only have research on two human trials looking at prostate cancer and low carbohydrate diets, with three clinical trials currently recruiting. Lin and colleagues recruited eight men with newly diagnosed prostate cancer and randomized them to a low fat, low glycemic load intervention arm (<20% fat & <100 glycemic load) or a “standard American” control arm (35% fat & >200 glycemic load) (22). The men on the low fat/low glycemic load diet lost more weight and showed significant gene expression changes in prostate epithelium on biopsy; suggesting the possibility of a therapeutic value. Many of these gene changes could conceivably alter the proliferation, metabolism and redox potential of prostate epithelial cells. In 2011, data was published from a cohort study of 566 Swedish men, which showed that adherence to a low carbohydrate, high protein diet was associated with a decreased risk of prostate cancer (23).
Breast Cancer:
Most of the research on carbohydrate restriction and breast cancer investigated its effects on prevention. Only two mouse model studies of breast cancer have evaluated a low carbohydrate diet and tumor growth. One study found that tumor growth was suppressed by a ketogenic diet (24). Another study showed that cancers grew slower in mice fed a low carbohydrate, high protein diet (25). A further reduction in growth in the primary tumor and metastasis was observed when the ketogenic diet was combined with a COX-2 inhibitor.
The Shanghai Women’s Health Study (included 79,942 Chinese women between the ages of 40-70) showed an association between a high carbohydrate, high glycemic load diet and an increased risk of pre-menopausal breast cancer (26). The Nurses’ Health Study (included 86,621 American women between the ages of 30-55) found lower risk for ER negative postmenopausal breast cancer with a diet high in fruits, vegetables, plant proteins, and plant fats with a total carbohydrate intake <50% and a glycemic load <100 (27).
The remaining studies looked at the ability of a low carbohydrate diet to lower metabolic markers associated with breast cancer risk. In one study, 115 overweight or obese women with increased risk for breast cancer followed either a calorie restricted, low carbohydrate diet (650 kcal, <50 grams carb x 2 days/week) or ad lib calorie, low carbohydrate diet (<50 grams carb x 2 days/week) or a 1500 kcal/day diet with normal carbohydrate intake for 7 days/week (28). They found that those in either of the carbohydrate restricted groups had greater weight loss, lower body fat, and less insulin resistance.
Another study showed similar results. Post-menopausal breast cancer survivors consumed a ketogenic diet (<40 grams carb, 800-1200 kcal) and successfully achieved weight loss and improvement in metabolic markers (lower CRP levels, lower fasting insulin levels, and lower circulating estrogen levels) (29). In another study, administration of a low-carbohydrate diet to patients with hormone responsive breast cancer on anti-estrogen or anti-aromatase therapy resulted in weight loss with improved lipid profiles and a decrease in signs of non-alcoholic hepatic steatosis (30). There is currently one active clinical trial looking further at a ketogenic diet in a breast cancer rehabilitation program.
Other Cancers:
Pediatric Astrocytoma
In 1995, a case study of two female pediatric patients with advanced stage astrocytoma showed a decrease in glucose uptake at the tumor site when following a ketogenic (10% carbohydrate) diet (31). Of the two, one continued the diet remaining free of disease progression for an additional 12 months.
Head and Neck Cancer
In head and neck cancer, it’s been hypothesized that carbohydrate restriction and ketosis can be supportive treatments by protecting normal tissue while sensitizing tumor tissue to radiation and chemotherapy and simultaneously supporting body and muscle mass maintenance (32). There is currently an active clinical trial looking at a low carbohydrate diet in head and neck cancer patients.
Endometrial Cancer
A study published in 2014 showed that endometrial cancer survivors following a ketogenic diet (<40 grams/day) had statistically significant decreases in weight, estrogenic markers, and fasting insulin levels thereby reducing their risk for recurrence (33).
Colon Cancer
One study found increasing risk for various colon cancers with increasing intake of fat, sucrose, lactose, glucose, and fructose (34), while no reduction in cancer risk was seen from following a 39% carbohydrate diet in a prospective cohort study of 62,582 Swedish people (35). Based on a review of the other studies referenced in this article, a 39% carbohydrate diet may not be considered low enough to elicit the benefits seen in some of the other studies.
Advanced or Metastatic Cancer
In the treatment of metastatic advanced cancers, a ketogenic diet has shown promise in mice models for reducing tumor growth and spread while prolonging survival (36, 37). In a human study of 10 patients, those with the highest degree of ketosis showed disease stabilization or partial remission, with no adverse effects (38). Another study of 16 patients following a ketogenic diet (<70 grams/day) showed improved emotional functioning and less insomnia in those who were able to maintain the diet (39). The remaining cancers have at least one mouse study available, but no human data looking at low carbohydrate diets and that specific type of cancer.
Melanoma
In mice with melanoma, researches have observed tumor inhibition at a 20% carb and 2% fat diet (40). And interestingly, when these same mice are given a phytonutrient supplement in addition to the low-carbohydrate or moderate-carbohydrate diet, they see even fewer tumor modules, plus increased survival times (41).
Liver
In mice with liver cancer, researchers have observed tumor inhibition and even regression when fed a 12% carb diet (42).
Lung, Pancreatic, Neuroblastoma
Mouse models have shown tumor inhibition, treatment enhancement, and prolonged survival in lung cancer, pancreatic cancer, and neuroblastoma (43, 44, 45, 46).
As mentioned throughout this section, there are numerous clinical trials currently underway looking at low carbohydrate diets and cancer. To stay up to date on current trials in this area, go to www.clinicaltrials.gov.
References:
1. Klement RJ. Carbohydrate restriction in cancer treatment and prophylaxis. Anticancer Research 2011;31:1987.
2. Klement RJ. Mimicking caloric restriction: what about macronutrient manipulation? A response to Meynet and Ricci. Trends in molecular medicine 2014;20:471-2.
3. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY) 2009;324:1029-33.
4. Maurer GD, Brucker DP, Bahr O, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011;11.
5. Chang H, Olson L, Schwartz K. Expression of ketolytic and glycolytic enzymes in malignant gliomas: Implication for ketogenic diet therapy. Journal of Neuropathology and Experimental Neurology 2013;72:565-6.
6. Rodriguez OC, Choudhury S, Kolukula V, et al. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell cycle (Georgetown, Tex) 2012;11:4436-46.
7. Scheck AC, Abdelwahab MG, Fenton KE, Stafford P. The ketogenic diet for the treatment of glioma: insights from genetic profiling. Epilepsy research 2012;100:327-37.
8. Marsh J, Mukherjee P, Seyfried TN. Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet. Nutrition & metabolism 2008;5:33.
9. Scheck AC, Abdelwahab M, Stafford P, et al. Mechanistic studies of the ketogenic diet as an adjuvant therapy for malignant gliomas. Cancer Research 2010;70.
10. Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutrition & metabolism 2007;4:5.
11. Abdelwahab MG, Woolf E, Fenton K, et al. Mechanistic analysis of the ketogenic diet versus KetoCal(registered trademark) as adjuvant treatments for malignant glioma. Cancer Research 2012;72.
12. Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutrition and Metabolism 2010;7.
13. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. Journal of neuro-oncology 2014;117:125-31.
14. Rieger J, Bahr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. International journal of oncology 2014;44:1843-52.
15. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: Report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer and Metabolism 2015;3.
16. Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008;68:11-9.
17. Kim HS, Masko EM, Poulton SL, et al. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU international 2012;110:1062-9.
18. Mavropoulos JC, Buschemeyer WC, 3rd, Tewari AK, et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer prevention research (Philadelphia, Pa) 2009;2:557-65.
19. Masko E, Thomas IJA, Antonelli JA, et al. Low-carbohydrate diets and prostate cancer growth: How low isnulllow enoughnull? Journal of Urology 2010;183:e554.
20. Caso J, Masko EM, Ii JAT, et al. The effect of carbohydrate restriction on prostate cancer tumor growth in a castrate mouse xenograft model. Prostate 2013;73:449-54.
21. Fokidis HB, Yieng Chin M, Ho VW, et al. A low carbohydrate, high protein diet suppresses intratumoral androgen synthesis and slows castration-resistant prostate tumor growth in mice. The Journal of steroid biochemistry and molecular biology 2015;150:35-45.
22. Lin DW, Neuhouser ML, Schenk JM, et al. Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: a feasibility study of using cDNA microarrays to assess the response to dietary intervention in target tissues. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2007;16:2150-4.
23. Ax E, Garmo H, Grundmark B, et al. Dietary patterns and prostate cancer risk: report from the population based ULSAM cohort study of Swedish men. Nutrition and cancer 2014;66:77-87.
24. Moulton CJ, Valentine RJ, Layman DK, et al. A high protein moderate carbohydrate diet fed at discrete meals reduces early progression of N-methyl-N-nitrosourea-induced breast tumorigenesis in rats. Nutrition & metabolism 2010;7:1.
25. Ho VW, Hamilton MJ, Dang NH, et al. A low carbohydrate, high protein diet combined with celecoxib markedly reduces metastasis. Carcinogenesis 2014;35:2291-9.
26. Wen W, Xiao OS, Li H, et al. Dietary carbohydrates, fiber, and breast cancer risk in Chinese women. American Journal of Clinical Nutrition 2009;89:283-9.
27. Fung T, Hu F, Hankinson S, Willett W, Holmes M. Low carbohydrate diets, DASH-style diet and risk of post-menopausal breast cancer. Cancer Research 2011;71.
28. Harvie M, Wright C, Pegington M, et al. Intermittent dietary carbohydrate restriction enables weight loss and reduces breast cancer risk biomarkers. Cancer Research 2011;71.
29. Olivo-Marston SE, Grainger E, Bittoni M, et al. Biomarkers of breast cancer risk in a randomized trial of a low-fat or low-carbohydrate weight loss intervention and physical activity among overweight and obese premenopausal women. Cancer Research 2012;72.
30. Manuela P, Elio R, Erika C, Editta B. Integrative approaches of diet and complementary medicine for the treatment of chemotherapy and hormone therapy side effects in patients with solid tumour: Experience in the hospital of Lucca (Italy). European Journal of Integrative Medicine 2012;4:15-6.
31. Nebeling LC, Lerner E. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. Journal of the American Dietetic Association 1995;95:693-7.
32. Klement RJ. Restricting carbohydrates to fight head and neck cancer-is this realistic? Cancer biology & medicine 2014;11:145-61.
33. Rojas-Espaillat LA, Krie AK, Demuth H, et al. Improved quality of life for early stage estrogen positive cancer survivors on a low-carbohydrate, calorie-restricted dietary intervention. Gynecologic Oncology 2014;133:204.
34. Hu J, la Vecchia C, Negri E, Mery L. Nutrients and risk of colon cancer. Cancers 2010;2:51-67.
35. Nilsson LM, Winkvist A, Johansson I, et al. Low-carbohydrate, high-protein diet score and risk of incident cancer; a prospective cohort study. Nutrition journal 2013;12:58.
36. Akgoc Z, Shelton LM, Ryan D, Zhu X, Seyfried TN. Restricted ketogenic diet reduces growth and distant organ metastasis in the murine VM-M3 metastatic tumor model. Cancer Research 2014;74.
37. Poff AM, Ward N, Seyfried TN, Arnold P, D’Agostino DP. Non-Toxic Metabolic Management of Metastatic Cancer in VM Mice: Novel Combination of Ketogenic Diet, Ketone Supplementation, and Hyperbaric Oxygen Therapy. PloS one 2015;10:e0127407.
38. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. A pilot safety and feasibility trial of a reduced carbohydrate diet in patients with advanced cancer. Journal of Clinical Oncology 2011;29.
39. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutrition & metabolism 2011;8:54.
40. Choi M, Lee J. The effect of carbohydrate-and fat-restricted diet on B16F10 melanoma model in C57BL6 mice: Focus on tumor growth inhibition and its underlying molecular target signaling pathways. European Journal of Cancer, Supplement 2010;8:67.
41. Choi M, Lee J. Synergic tumor growth suppression with carbohydrate-restriction diet and natural AMP-dependent protein kinase activators. European journal of cancer 2014;50:76.
42. Rudolph I, Kettelhake A, Mastrobuoni G, Kempa S, Cramer T. Progression and therapy efficacy of liver cancer is determined by macronutrient composition. Oncology Research and Treatment 2014;37:54.
43. Allen BG, Bhatia SK, Buatti JM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19:3905-13.
44. Allen BG, Fath MA, Simons AL, Bhatia SK, Buatti JM, Spitz DR. Enhancing tumor chemo-radio-sensitization using ketogenic diets. International Journal of Radiation Oncology Biology Physics 2010;78:S114-S5.
45. Shukla SK, Gebregiworgis T, Purohit V, et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer & metabolism 2014;2:18.
46. Morscher RJ, Aminzadeh-Gohari S, Feichtinger R, et al. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-nu mouse model. PloS one 2015;10.
Legal notice
This documentation has been compiled by the UCSF Osher Center with all due care and expert knowledge. However, the UCSF Osher Center provides no assurance, guarantee or promise with regard to the correctness, accuracy, up-to-date status or completeness of the information it contains. Readers are strongly advised to discuss the information with their physician. Accordingly, the UCSF Osher Center shall not be liable for damage or loss caused because anyone relies on the information.

